- Joined
- Jan 31, 2021
I had an argument with friends regarding my 11yr old niece schoolwork. I want to teach her how to do this correctly.
My understanding is that (2) is correct since the question is trying to get the student to understand that 'reading the thermometer is the way to tell whether is water boiling'.
But some of my friends are trying to argue that (4) is correct too since when water boils, bubbles appear.
What do kiwifarmers think? am I right or do their statement holds water (no pun intended)? and what will be a good way to teach a child to tackle similar questions like these?
I attached the question below, the teacher marked (4) as wrong and (2) as correct.
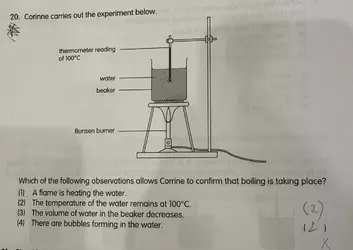
My understanding is that (2) is correct since the question is trying to get the student to understand that 'reading the thermometer is the way to tell whether is water boiling'.
But some of my friends are trying to argue that (4) is correct too since when water boils, bubbles appear.
What do kiwifarmers think? am I right or do their statement holds water (no pun intended)? and what will be a good way to teach a child to tackle similar questions like these?
I attached the question below, the teacher marked (4) as wrong and (2) as correct.

