- Joined
- Nov 16, 2021
Technically any time you burn hydrocarbons, you are producing new water. Hydrocarbon + O2 = xC02 + yH2O moleculesYeah and the formula for water is H2O yet we don't have artificial water (AFAIK). I don't think chemistry is always as simple as mixing one part X with three parts Y and getting the desired chemical. At least not with equipment we can get for home use.
Maybe I'm wrong and it is that simple, but if that's the case why don't we have artificial water? Wouldn't companies like Nestlé be using that instead of buying water rights everywhere? Wouldn't we have fewer effective droughts because we could just spray artificial water everywhere in dry weather?
The bigger issue with organic compounds is that the formula isn't enough, the way the carbon bonds are structured matters too.
PubChem lists 4,451 results for the Chemical Formula C18h24o2
from estradiol::
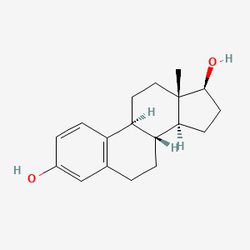
to Nimbiol
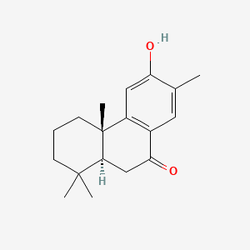
to
8,10,12-Octadecatriynoic acid
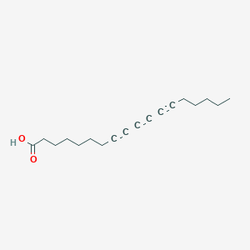
These all have the same chemical formula. And it's not like you're assembling molecules one at a time like lego, you have to trigger the right reactions with the right precursor molecules so that the same structures occur naturally as part of the reaction. It's not like you can just mix 18 parts carbon to 24 parts hydrogen and 2 part oxygen and expect the atoms to all group together. Saying the chemical formula is actually "quite simple" is absurd, and makes me thing the person isn't actually a chemistry major.
Last edited:











